Chinese Medicine Regulatory Office
Site Menu
Good Manufacturing Practice (GMP) for Proprietary Chinese Medicines

GMP: the gold standard of quality
Good Manufacturing Practice (GMP) is a quality assurance system widely adopted by the drug manufacturing industry worldwide.
GMP ensures the production of safe and consistent quality products through establishing standards for drug manufacturing hardware and software, such as raw materials, premises, equipment, sanitation, personnel training and quality management.
Department of Health

What are the benefits of implementing GMP for proprietary Chinese medicines?
Diminish the risks inherent in any proprietary Chinese medicines manufacturing processes, e.g. cross-contamination of ingredients, false labels being put on containers, etc., thus safeguard public health by ensuring the quality, safety and efficacy of proprietary Chinese medicines being manufactured comply with requirements.
Promote the standardization and internationalization of the proprietary Chinese medicines manufacturing industry, and boost the public confidence in using proprietary Chinese medicines.
A proprietary Chinese medicine refers to any proprietary product composed solely of Chinese herbal medicines, or any materials of herbal, animal or mineral origin customarily used by the Chinese as active ingredients, formulated in a finished dose form and known or claimed to be used for the diagnosis, treatment, prevention or alleviation of any disease or any symptom of a disease in human beings, or for the regulation of the functional states of the human body.
How to identify the proprietary Chinese medicines manufactured by GMP manufacturers?
Manufacturers with GMP certificate may label their products with ‘GMP’ to indicate that their products are manufactured in accordance with GMP standard.

Legal requirements for implementation of GMP for proprietary Chinese medicines in Hong Kong
According to Section 133 of the Chinese Medicine Ordinance (Chapter 549 of the Laws of Hong Kong), any licensed proprietary Chinese medicines manufacturer may apply to the Chinese Medicines Board under the Chinese Medicine Council of Hong Kong for a certificate for manufacturer to certify that they follow the requirements of good practices in manufacture and quality control of proprietary Chinese medicines.
Certificate for Manufacturer (GMP)
In view of the special nature of proprietary Chinese medicines, the Chinese Medicines Board under the Chinese Medicine Council of Hong Kong issued the ‘Hong Kong Good Manufacturing Practice Guidelines for Proprietary Chinese Medicines’ in 2003 to provide guidance to proprietary Chinese medicines manufacturers.
Proprietary Chinese medicines manufacturers should meet the requirements of the ‘Hong Kong Good Manufacturing Practice Guidelines for Proprietary Chinese Medicines’ for issuance of GMP certificate
How to obtain the list of proprietary Chinese medicines GMP manufacturers in Hong Kong?
- Access the website of the Chinese Medicine Council of Hong Kong (website: www.cmchk.org.hk)
- Make enquiries at the Chinese Medicine Regulatory Office of the Department of Health (Tel. No.: 2319 5119)

Key elements of Good Manufacturing Practice for Proprietary Chinese Medicines
- Quality Management: An appropriate infrastructure (organizational structure, procedures, processes and resources) and ‘quality assurance’ measures taken to ensure that a product will satisfy defined quality requirements.
- Personnel: There must be sufficient qualified personnel to carry out relevant duties.
- Premises: Premises must be located, designed, constructed, adapted and maintained to suit the manufacturing processes to be carried out.
- Equipment: Equipment must be located, designed, constructed, adapted and maintained to comply with the requirements of the manufacturing processes of proprietary Chinese medicines to be carried out.
- Documentation: Documentation is a fundamental part of the quality assurance system and, as such, should be related to all aspects of the requirements of GMP.
- Manufacture: Manufacturing processes must follow clearly defined procedures and comply with the principles of GMP and the conditions stated in the manufacturer licence in proprietary Chinese medicines.

Key elements of Good Manufacturing Practice for Proprietary Chinese Medicines
- Validation: Validation is the documented act of proving that any procedure, manufacturing process, equipment, material, activity or system actually leads to the expected results.
- Quality Control: Quality control is concerned with sampling, making specifications and testing, which ensures that necessary and relevant tests are carried out, and only materials and products with satisfactory quality are released.
- Contract Manufacture and Test: Contract manufacture and test must be clearly defined and managed, to avoid any misunderstandings between the parties to the contract that could affect the quality of the product, manufacture operations or tests.
- Complaints: All complaints and other information concerning potentially defective products must be carefully investigated, according to written procedures.
- Product Recalls: There should be a product recall system to recall from the market, promptly and effectively, products known or suspected to be defective. The Chinese Medicines Board under the Chinese Medicine Council of Hong Kong should be notified before the product recall starts.
- Self-Inspection/Quality Audits: The purpose of self-inspection is to evaluate the manufacturer’s compliance with GMP requirements in all aspects of manufacture and quality control.
Relevant information on the ‘Hong Kong Good Manufacturing Practice Guidelines for Proprietary Chinese Medicines’ can be downloaded from the website of the Chinese Medicine Council of Hong Kong (website: /www.cmchk.org.hk)

How to apply for a Certificate for Manufacturer?
Application Requirements
- Licensed manufacturers in proprietary Chinese medicines;
- The manufacturers must prove that they comply with the “Hong Kong Good Manufacturing Practice Guidelines for Proprietary Chinese Medicines” in manufacture and quality control of proprietary Chinese medicines; and
- The applicants must be holders of the licence or persons authorized by the companies
How to obtain an application form?
- Obtain in person from the Chinese Medicine Regulatory Office of the Department of Health; or
- Download from the website of the Chinese Medicine Council of Hong Kong (website: www.cmchk.org.hk)
To have a better understanding in GMP for pCm
- Visiting the GMP for Proprietary Chinese Medicines Web Resources Platform(website:www.cmro.gov.hk/html/eng/gmpweb/index.html)
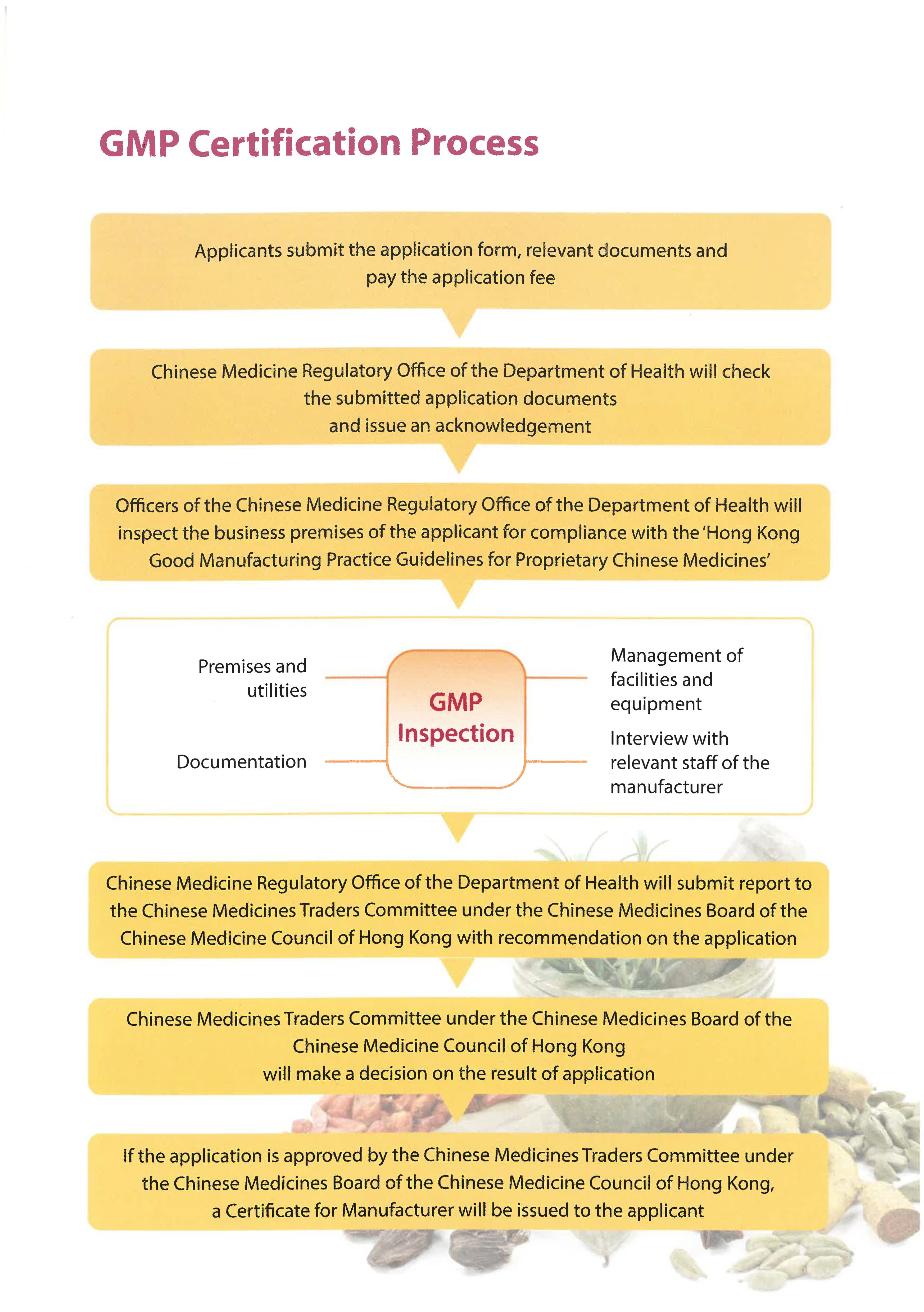
GMP Certification Process
- Applicants submit the application form, relevant documents and pay the application fee
- Chinese Medicine Regulatory Office of the Department of Health will check the submitted application documents and issue an acknowledgement
- Officers of the Chinese Medicine Regulatory Office of the Department of Health will inspect the business premises of the applicant for compliance with the ‘Hong Kong Good Manufacturing Practice Guidelines for Proprietary Chinese Medicines’
- GMP Inspection
- Premises and utilities
- Management of facilities and equipment
- Documentation
- Interview with relevant staff of the manufacturer
- Chinese Medicine Regulatory Office of the Department of Health will submit report to the Chinese Medicines Traders Committee under the Chinese Medicines Board of the Chinese Medicine Council of Hong Kong with recommendation on the application
- Chinese Medicines Traders Committee under the Chinese Medicines Board of the Chinese Medicine Council of Hong Kong will make a decision on the result of application
- If the application is approved by the Chinese Medicines Traders Committee under the Chinese Medicines Board of the Chinese Medicine Council of Hong Kong, a Certificate for Manufacturer will be issued to the applicant

Any International Standards for GMP?
In addition to the World Health Organization (WHO) GMP guidelines, the Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme (PIC/S) GMP guidelines are widely adopted by the drug manufacturing industry worldwide.
- WHO World Health Organization GMP (website: www.who.int)
- PIC/S Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme GMP (website: www.picscheme.org)
Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme (PIC/S)
The mission of PIC/S is “to lead the international development, implementation and maintenance of harmonised Good Manufacturing Practice (GMP) standards and quality systems of inspectorates in the field of medicinal products”. There are many participating authorities in PIC/S, including GMP inspectorates of majority countries of the European Union, Australia and Singapore, which involve the inspection of manufacturers of herbal medicinal products.
Chinese Medicine Regulatory Office, Department of Health January 2020
The information in this pamphlet may be re-disseminated or reproduced, provided that the Chinese Medicine Regulatory Office (CMRO), as the source of information, is acknowledged and that the re-dissemination or reproduction is for non-commercial use.
Any other reproduction, adaptation, distribution, dissemination or making available of the information in this pamphlet for commercial use is strictly prohibited unless prior written authorization is obtained from the CMRO.