Chinese Medicine Regulatory Office
Site Menu
What you should know when purchasing proprietary Chinese medicines

What you should know when purchasing proprietary Chinese medicines
To safeguard public health, the Chinese Medicine Ordinance provides for a registration system for proprietary Chinese medicines which stipulates that all proprietary Chinese medicines manufactured or sold in Hong Kong must be registered.

What is proprietary Chinese medicine?
•A proprietary Chinese medicine refers to any proprietary product composed solely of any Chinese herb as the active ingredient, formulated in a finished dose form and known or claimed to be used for the diagnosis, treatment, prevention or alleviation of any disease, regulation of the functional states of the human body.
Registration requirements of proprietary Chinese medicines
•To be approved for registration, a proprietary Chinese medicine must meet the registration requirements for safety, quality and efficacy set by the Chinese Medicines Board of the Chinese Medicine Council of Hong Kong.

Label of a registered proprietary Chinese medicine
•The container or package of a registered proprietary Chinese medicine must be properly labeled with the required items. You may choose a proprietary Chinese medicine suitable to your needs according to these particulars. But you should consult a Chinese medicine practitioner in case of doubt.
The label should indicate:
name;
major active ingredients;
dosage;
method of usage;
expiry date;
place of origin;
registration number;
name of holder of the certificate of registration;
packing specification and batch number in respect of that medicine
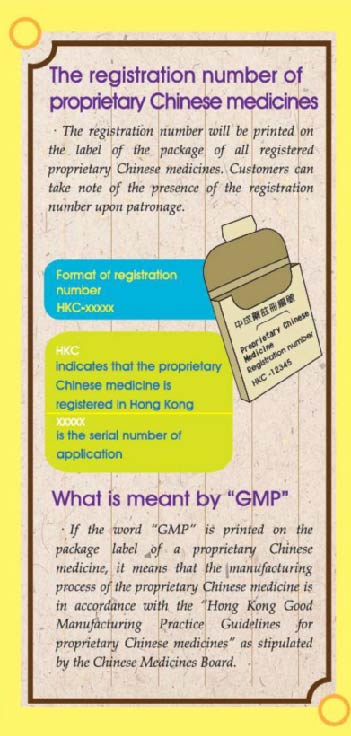
The registration number of proprietary Chinese medicines
•The registration number will be printed on the label of the package of all registered proprietary Chinese medicines. Customers can take note of the presence of the registration number upon patronage.
Format of registration number: HKC-xxxxx
HKC indicates that the proprietary Chinese medicine is registered in Hong Kong;
xxxxx is the serial number of application.
What is meant by "GMP"
•If the word "GMP" is printed on the package label of a proprietary Chinese medicine, it means that the manufacturing process of the proprietary Chinese medicine is in accordance with the "Hong Kong Good Manufacturing Practice Guidelines for proprietary Chinese medicines" as stipulated by the Chinese Medicines Board.
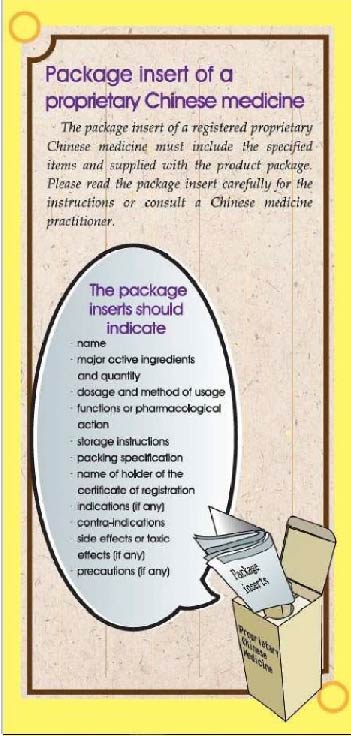
Package insert of a proprietary Chinese medicine
•The package insert of a registered proprietary Chinese medicine must include the specified items and supplied with the product package. Please read the package insert carefully for the instructions or consult a Chinese medicine practitioner.
The package inserts should indicate:
name;
major active ingredients and quantity;
dosage and method of usage;
functions or pharmacological action;
storage instructions;
packing specification;
name of holder of the certificate of registration;
indications (if any);
contra-indications
side effects or toxic effects (if any);
precautions (if any)

Transitional registration for proprietary Chinese medicines
•A proprietary Chinese medicine, which was sold or manufactured in Hong Kong on 1 March 1999, is eligible for transitional registration provided that an application is made by the relevant manufacturer, importer, or a local representative / agent of the manufacturer during the specified period to the Chinese Medicines Board.
•The transitional registration number of a proprietary Chinese medicine will be printed on its package label. Information regarding safety, quality and efficacy of the proprietary Chinese medicine must be submitted to the Chinese Medicines Board for assessment prior to formal registration.
Format of transitional registration number: HKP-xxxxx
HKP means that the proprietary Chinese medicine is transitionally registered in Hong Kong;
xxxxx represents the serial number of application
Chinese Medicine Regulatory Office
Department of Health
Website: www.cmro.gov.hk